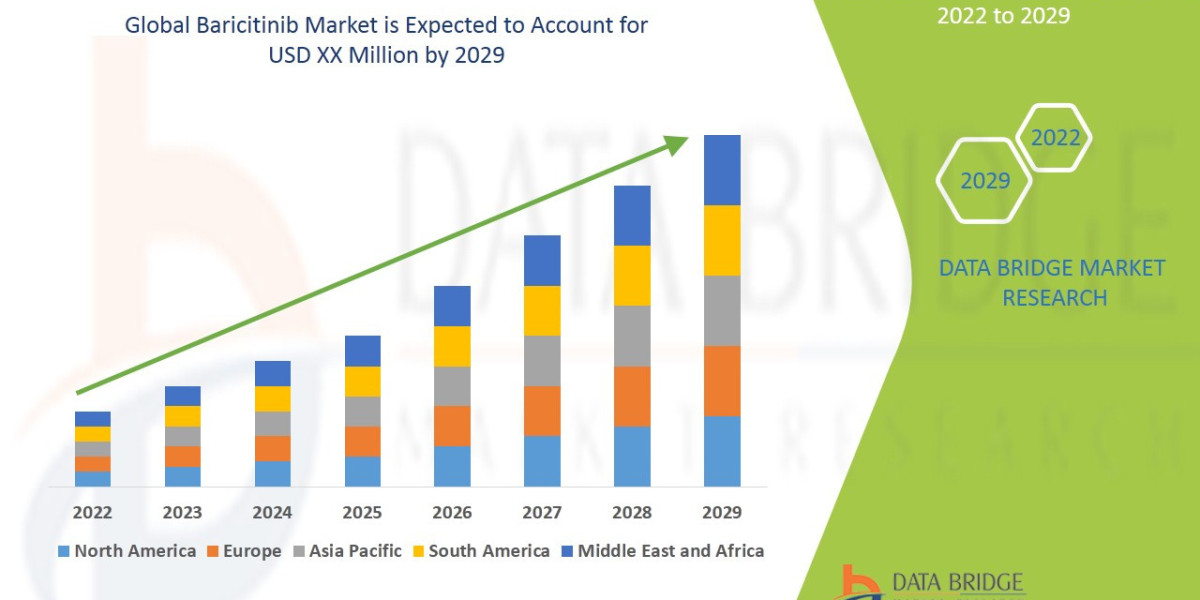
The baricitinib market is expected to witness market growth at a rate of 11.90% in the forecast period of 2022 to 2029.
Baricitinib, a small-molecule Janus kinase (JAK) inhibitor sold most widely under the brand name Olumiant (Eli Lilly/Incyte), has evolved from a rheumatoid-arthritis therapy into a multi-indication platform with growing commercial footprint. Beyond its original approval for moderate-to-severe rheumatoid arthritis, baricitinib’s regulatory gains (including approval for severe alopecia areata and emergency use in certain COVID-19 settings) and broader clinician familiarity have raised its strategic importance for manufacturers, payers, and patients. These clinical and regulatory shifts—paired with looming patent expirations and geographic manufacturing partnerships—are reshaping competitive dynamics and access worldwide.
Access The Report: https://www.databridgemarketresearch.com/reports/global-baricitinib-market
Market Trends
Indication diversification. Baricitinib’s migration from RA into dermatology (alopecia areata) and acute care (hospitalized COVID-19) has materially expanded its addressable market. Indication expansions boost prescriber awareness, catalyze payer reviews, and justify lifecycle investments such as new formulations and combination studies.
Geographic localization of manufacturing. Strategic licensing and local manufacturing deals (for example, agreements to enable regional production in Africa) are being used to reduce supply risk, lower cost, and facilitate market access in lower- and middle-income countries—a model likely to repeat in other emerging markets.
Reuters
Patent cliff and generic planning. Key patents protecting baricitinib are scheduled to expire in stages across major jurisdictions (U.S. patents around 2030, EU close to 2032, Japan later), creating a predictable window for generic entrants and biosimilar-style price competition. Innovator firms are responding with lifecycle management: filing for new indications, reformulations, and regional sublicensing to sustain revenue.
Policy and safety scrutiny. JAK inhibitors as a class have faced heightened regulatory attention over safety signals; this scrutiny affects label language, payer reimbursement criteria, and physician prescribing patterns—especially in populations at higher baseline risk for infections or thrombosis. Regulatory risk management will remain a constant theme.
Market Size
Estimates for the global baricitinib market vary by source and scope (which indications and revenue streams are included), but consensus points to a multi-billion-dollar market that expanded substantially in the early 2020s. Various market research models place 2023–2024 market value in the range of roughly USD 1.2–2.9 billion depending on assumptions about COVID-related demand and alopecia uptake, with forecasts projecting strong compound annual growth into the early 2030s. Growth projections to 2030–2035 range from mid-single digits to double-digit CAGRs in different reports—driven primarily by indication expansion, geographic rollouts, and price/mix dynamics.
Concrete commercial reporting from Eli Lilly also underscores the product’s material contribution: Olumiant sales have been rising year-on-year, with reported quarterly increases and cumulative annual revenues reaching several hundred million dollars and approaching a billion in broader reporting windows—evidence of both established RA demand and newer indication uptake.
Market Share
Eli Lilly remains the dominant commercial presence in the baricitinib market through its Olumiant brand; incumbent market share reflects first-mover advantage, marketed indications, and global distribution networks. Market-share splits between brand and future generic entrants will depend on patent outcomes, the pace of generic approvals, and regional tendering practices. In markets where Lilly pursues sublicensing or local manufacturing partnerships, incumbent share may be preserved longer through authorized generics or co-supply arrangements. Independent smaller suppliers of active pharmaceutical ingredient (API) and manufacturers preemptively positioning for generic launches are already on the radar, signaling that market share erosion is likely once exclusivity sunsets.
Market Growth
Expanded indications (alopecia areata, COVID-19 acute care) increase treated population and average treatment duration.
Rising diagnosis rates and improved access to specialty care in emerging markets push absolute patient numbers higher.
Price optimization and reimbursement negotiations as manufacturers secure formulary access across geographies.
Strategic partnerships for local production accelerate roll-out in price-sensitive regions.
Reuters
Patent expirations will invite generic competition and downward pricing pressure in the coming decade.
Safety perceptions and regulatory restrictions on JAK inhibitors may limit adoption in certain patient subgroups or trigger tighter label warnings.
Reimbursement variability—payers may restrict access to approved indications unless cost-effectiveness or long-term outcome data are compelling.
Manufacturing and supply chain risks (API shortages, geopolitics) can constrain growth in short windows, though localization deals are mitigating this risk.
Market Demand
Demand for baricitinib is shaped by clinical need, treatment guidelines, and the balance between efficacy, safety, and cost. In rheumatoid arthritis, baricitinib competes with biologics and other oral JAK inhibitors; its favorable oral route and competitive efficacy make it an attractive option for many clinicians and patients. In alopecia areata, regulatory approval filled a large unmet-need gap—creating a new and high-value demand pool because it is the first systemic therapy approved specifically for severe disease in adults. Temporary spikes in demand during the COVID-19 pandemic also showed how acute-care emergency authorizations can influence short-term volumes.
Demand elasticity will vary by region: high-income markets will drive premium pricing and managed-care negotiation, while lower-income markets will rely on generics or licensed local manufacturing to unlock volume. Patient and clinician education, plus real-world evidence demonstrating long-term safety and effectiveness in expanded indications, will be decisive for sustained demand growth.
Browse More Reports:
Global Udder Health Market
Global Vascular Ultrasonography Market
Global Video Conferencing Systems Market
Global Vitamin Patches Market
Global Watches Market
Global Wearable Sensors Market
Global Weight Management Diet Market
Global Xeroderma Pigmentosum Treatment Market
Global X-Ray System Market
Global Yacht Charter Market
Global Coagulation Factor VII Treatment Market
Global Compound Fertilizer Market
Global Hydrocarbons Market
Global Label Printer Market
Global Agricultural Fertigation and Chemigation Market
Market Future Insights
Generic entry will be the defining inflection. As key patents expire (U.S. ~2030; EU ~2032; Japan slightly later), expect rapid generic launches in price-sensitive markets and tender-driven procurement shifts in public systems. Innovator revenues will increasingly depend on new indications, patented formulations, and authorized generics or licensing models to preserve margins.
Value-based contracting and indication-level pricing. Payers will likely push for outcome-based agreements—especially for alopecia areata where cosmetic and quality-of-life outcomes are harder to quantify—and for step-therapy approaches in chronic indications. Manufacturers that present robust real-world effectiveness and health-economic models will retain negotiating leverage.
Regional manufacturing as access lever. Partnerships that localize production (e.g., the Lilly–Eva Pharma arrangement for Africa) will accelerate access, reduce cost of goods, and potentially blunt generic price erosion in those territories through authorized local supply agreements. Similar south-south licensing deals will likely proliferate.
Portfolio strategies and combination research. Expect continued investment in combination therapies and new formulations (longer-acting, once-daily optimizations) and in exploring synergies with other immunomodulators—both to expand clinical utility and to create patentable follow-on assets.
Safety and regulatory vigilance. Ongoing post-marketing surveillance and comparative safety studies will influence guideline placement and patient selection, which in turn affects market uptake curves across segments.
Conclusion
Baricitinib stands at an inflection point: clinical expansion and rising sales have turned it into a multi-billion-dollar opportunity, while impending patent expiries and safety/regulatory dynamics will reshape how value is captured. The winners in this market will be those who balance evidence-driven indication expansion, smart lifecycle management, regional access partnerships, and payer-friendly value propositions. For investors, payers, and manufacturers, the near term promises continued growth; the next decade will determine who sustains premium positioning and who pivots to compete in a generics-dominated landscape.
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Baricitinib Market, Baricitinib Market Trends, Baricitinib Market Growth, Baricitinib Market Demand, Baricitinib Market Size, Baricitinib Market Scope, Baricitinib Market Insights, Baricitinib Market Analysis,