Executive Summary
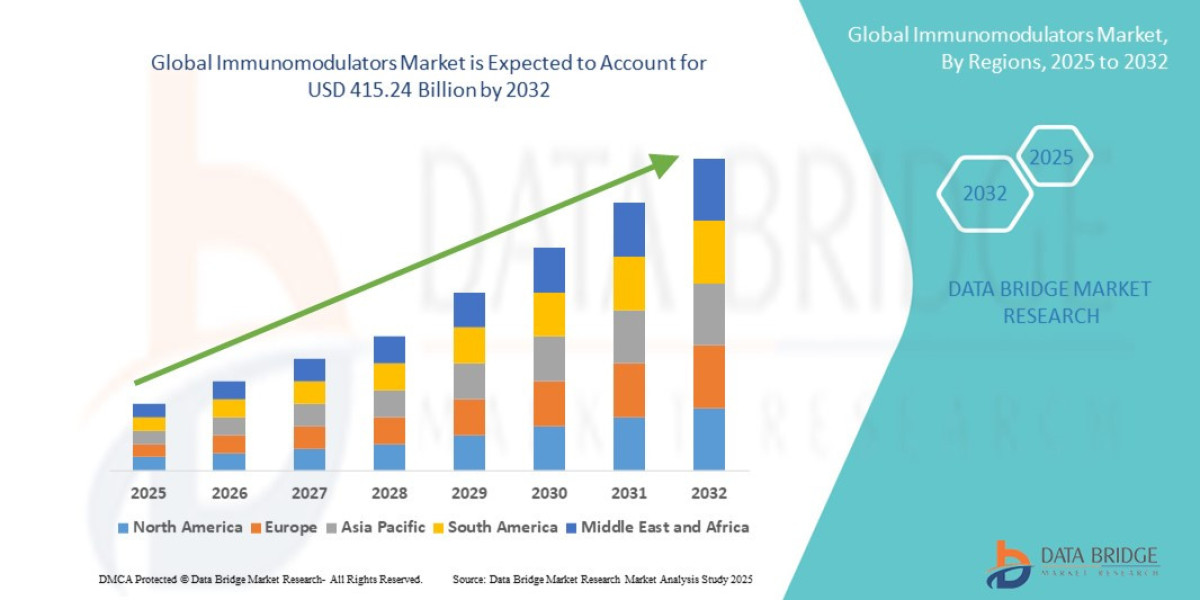
- The global immunomodulators market size was valued at USD 247.16 billion in 2024 and is expected to reach USD 415.24 billion by 2032, at a CAGR of 6.70% during the forecast period
Market Overview
Immunomodulators are a diverse class of drugs designed to regulate or modify the immune system's response, either by stimulating (immunostimulants) or suppressing (immunosuppressants) its activity. Their therapeutic utility spans a broad range of conditions where the immune system is either underactive, overactive, or misdirected.
Key Segments
The market is primarily segmented by product type and application:
By Product Type:
Immunosuppressants: This segment, which includes monoclonal antibodies (mAbs), calcineurin inhibitors, and glucocorticoids, historically holds the dominant market share (often over 50%). Their primary use is in organ transplantation (to prevent rejection) and the management of autoimmune diseases like rheumatoid arthritis, psoriasis, and multiple sclerosis.
Immunostimulants: This fastest-growing segment is driven by their pivotal role in oncology (e.g., immune checkpoint inhibitors like PD-1/PD-L1 and CAR-T cell therapies) and infectious diseases (e.g., vaccine adjuvants).
By Application:
Oncology: The largest application segment, where immunomodulators like checkpoint inhibitors have revolutionized cancer treatment, shifting the therapeutic focus from cytotoxic protocols to immune activation.
Autoimmune & Inflammatory Diseases: The traditional stronghold, covering indications like Multiple Sclerosis (MS), Rheumatoid Arthritis (RA), and Inflammatory Bowel Disease (IBD).
Others: Includes HIV, respiratory diseases, and organ transplantation.
Market Drivers
Rising Disease Burden: The increasing global prevalence of chronic illnesses, particularly autoimmune disorders and various cancers, is the fundamental demand-side driver.
Technological Advancements in Biologics: Continuous innovation in the development of highly specific monoclonal antibodies and novel cell and gene therapies provides superior efficacy and safety profiles compared to older, non-specific therapies.
Growing Adoption of Immunotherapy: The shift in clinical guidelines, particularly in oncology, that favor targeted immunotherapy as first-line and combination treatments is a major growth catalyst.
Increasing Healthcare Expenditure: Higher spending on specialty drugs in developed economies supports the premium pricing of novel immunomodulatory biologics.
Market Size & Forecast
- The global immunomodulators market size was valued at USD 247.16 billion in 2024 and is expected to reach USD 415.24 billion by 2032, at a CAGR of 6.70% during the forecast period
For More Information visit https://www.databridgemarketresearch.com/reports/global-immunomodulators-market
Key Trends & Innovations
The market's future is being shaped by several key technological and commercial trends that emphasize precision, patient compliance, and market accessibility.
Precision and Next-Generation Modalities
Shift to Pathway-Specific Targeting: Research is moving away from disease-specific treatments toward modulating core immune pathways. Novel targets like TYK2 and FcRn inhibitors are gaining traction, offering therapies that could treat multiple indications with reduced systemic side effects. For example, TYK2 inhibitors represent a breakthrough in oral small-molecule immunomodulators for conditions like psoriasis.
Bispecific and Trispecific Antibodies: Next-generation biologics are evolving beyond traditional monoclonal antibodies. Bispecific antibodies (bsAbs), which can bind to two different antigens simultaneously, are designed to enhance T-cell engagement and improve efficacy, particularly in complex solid tumors.
AI-Enabled Drug Discovery: The application of Artificial Intelligence (AI) is accelerating the identification of novel immunomodulatory targets and optimizing the design of new molecules, promising faster development timelines and higher success rates in preclinical phases.
Commercial and Regulatory Dynamics
Biosimilar Disruption: The patent cliff for blockbuster biologics, such as Humira and Stelara, has led to a major influx of biosimilars. This disruption is a double-edged sword: it introduces significant price elasticity and affordability for payers and patients, but it also creates immense revenue pressure on originator companies, forcing them to rapidly innovate their pipelines.
Focus on Oral and Subcutaneous Formulations: There is a significant trend towards developing immunomodulators with enhanced patient convenience and compliance. Oral small molecules and subcutaneous auto-injector formulations are challenging the dominance of high-risk intravenous infusions, which shifts the site of care away from hospitals to home-care settings.
Companion Diagnostics (CDx): The adoption of CDx biomarkers is crucial for precision dosing and patient selection, especially in oncology (e.g., PD-L1 testing). This trend ensures that high-cost therapies are delivered to the patients most likely to respond, optimizing both clinical outcomes and healthcare spending.
Competitive Landscape
The immunomodulators market is a highly consolidated and intensely competitive arena, dominated by a few large, multinational pharmaceutical and biotechnology firms. Competition revolves around pipeline innovation, intellectual property, and market access strategies.
Major Players and Strategic Focus
| Company (Tier 1) | Key Product Areas | Strategic Focus |
| AbbVie Inc. | Anti-TNF α (Humira), IL-23 (Skyrizi) | Defensive strategies against biosimilars, and portfolio diversification into next-gen oral and injectable biologics. |
| F. Hoffmann-La Roche AG | Anti-CD20 (Rituxan/Rituximab), Checkpoint Inhibitors (Tecentriq) | Dominance in oncology and autoimmune segments; R&D in cell and gene therapy and novel immune targets. |
| Bristol-Myers Squibb (BMS) | Checkpoint Inhibitors (Opdivo), Immunomodulatory Drugs (Pomalyst) | Leader in immuno-oncology (IO) and hematology; focused on combination IO therapies. |
| Johnson & Johnson | Anti-IL-12/23 (Stelara), Anti-CD38 (Darzalex) | Portfolio expansion through M&A and pipeline development in inflammatory diseases and multiple myeloma. |
| Pfizer Inc. | JAK Inhibitors (Xeljanz), Biologics | Strategic focus on diversifying into specialty immunology and managing the transition from small molecules to biologics. |
Competitive Strategies
Pipeline Depth and Diversification: Companies are aggressively investing in R&D to maintain a deep pipeline of novel assets, focusing on new mechanisms of action to offset the revenue erosion from biosimilars.
Mergers & Acquisitions (M&A) and Collaborations: Strategic acquisitions of smaller biotech firms with innovative platforms (e.g., CAR-T, novel cytokine pathways) are common to quickly onboard cutting-edge technologies and secure intellectual property.
Life Cycle Management (LCM): Extending the exclusivity of existing blockbusters through the development of new formulations (e.g., high-concentration, citrate-free), new delivery devices, and securing additional indications.
Regional Insights
The market exhibits distinct regional dynamics driven by healthcare infrastructure, regulatory policies, and disease prevalence.
North America (Dominant)
Market Share: Holds the largest share (often over 38%), driven by the United States.
Drivers: High prevalence of chronic diseases, a robust healthcare infrastructure, substantial R&D investment (both public and private), favorable reimbursement policies, and a rapid adoption of premium-priced novel therapies. The FDA’s streamlined approval process for novel biologics further accelerates market penetration.
Europe (Mature)
Market Share: The second-largest market.
Dynamics: Characterized by established healthcare systems but faces greater pricing pressure from governments and the accelerated introduction of biosimilars, particularly following the loss of exclusivity for major anti-TNF α drugs. Favorable health reimbursement for innovative therapies in key markets like Germany and the UK supports growth.
Asia-Pacific (Fastest Growth)
Growth Rate: Projected for the highest CAGR (often over 7%).
Opportunities: Driven by an aging population, rising incidence of cancer and autoimmune diseases, improving healthcare access, increasing disposable income, and supportive government initiatives for local drug manufacturing and R&D (especially in China and India). This region represents a critical future revenue driver for multinational firms.
Challenges & Risks
Despite the buoyant growth, the immunomodulators market is subject to significant challenges.
High Cost of Biologics and Reimbursement Hurdles: The premium price tag of complex biologic immunomodulators creates barriers to access, leading to stringent reimbursement policies, prior authorization requirements, and step therapy protocols from payers.
Safety and Adverse Effects: Immunomodulatory drugs, particularly immunosuppressants and some immuno-oncology agents, carry risks of serious adverse reactions, including increased susceptibility to infections, secondary malignancies, and potential for autoimmune side effects (e.g., Cytokine Release Syndrome or immune-related adverse events).
Limited Understanding of Immune Complexity: The intricate and highly variable nature of the human immune system remains partially understood. This knowledge gap makes developing highly selective and universally efficacious modulators challenging, leading to high failure rates in clinical trials and protracted R&D timelines.
Intellectual Property (IP) and Biosimilar Erosion: The impending or actual expiration of patents for market-leading drugs exposes originator companies to significant revenue loss from generic and biosimilar competition.
Opportunities & Strategic Recommendations
The challenges simultaneously define the opportunities for savvy stakeholders.
Opportunities
Personalized/Stratified Medicine: The greatest opportunity lies in leveraging genomic and proteomic biomarkers to identify patient subpopulations most likely to benefit from a specific immunomodulator, thus maximizing therapeutic value and minimizing adverse effects.
Oral Small Molecules with Biologic Efficacy: Developing next-generation small-molecule drugs that can target specific immune pathways with the efficacy of biologics but offer the convenience and potentially lower manufacturing cost of oral administration.
Combination Therapies: The future of immuno-oncology and autoimmune treatment is in combination regimens. Investing in R&D for effective synergistic combinations of immunomodulators with other drug classes (e.g., chemotherapy, targeted therapy) will unlock new treatment paradigms.
Expansion into Emerging Markets: Aggressive but tailored market entry strategies for the Asia-Pacific and Latin American markets, which require navigating local regulatory environments and addressing demand for cost-effective therapies.