Executive Summary
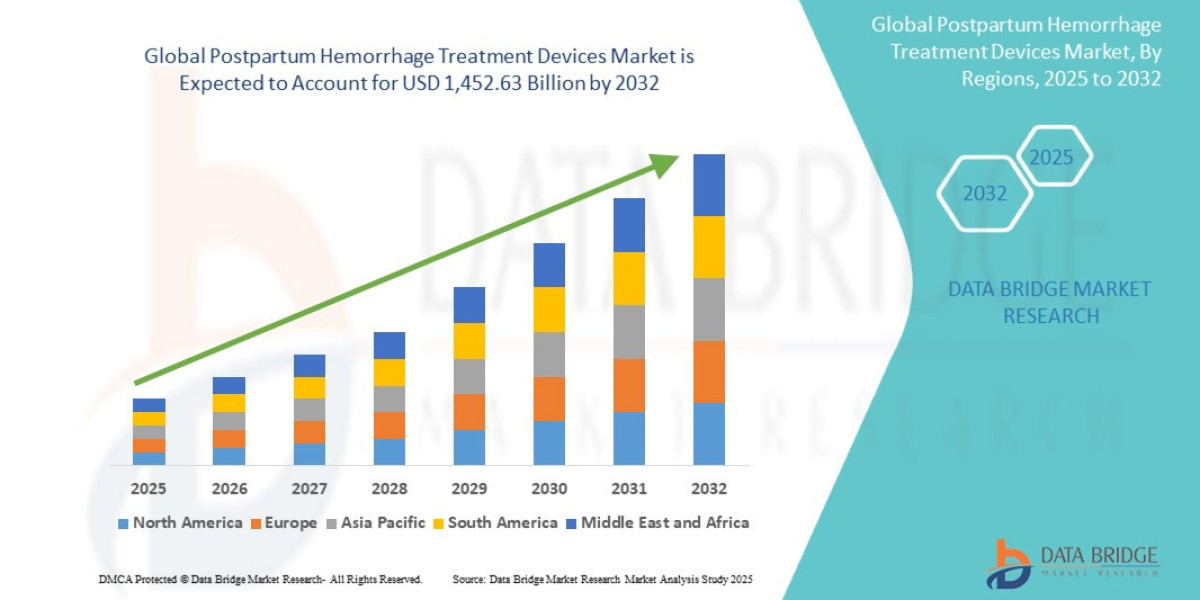
- The global postpartum hemorrhage treatment devices market was valued at USD 997.65 million in 2024 and is expected to reach USD 1,452.63 million by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 4.9%,
Market Overview
Definition and Therapeutic Context
The Postpartum Hemorrhage Treatment Devices Market encompasses all non-pharmacological, mechanical, and specialized surgical instruments utilized to control excessive bleeding in the immediate period following delivery. PPH is clinically defined as blood loss greater than 500 mL (vaginal birth) or 1,000 mL (C-section). Devices are typically classified as second-line or third-line interventions in PPH management protocols, used to stabilize the patient, reduce blood loss, and buy time before more definitive surgical or interventional radiological procedures are performed. The primary cause addressed by mechanical devices is uterine atony (failure of the uterus to contract).
Key Market Segments by Product Type
Uterine Balloon Tamponade (UBT) Systems (Leading Segment): These devices are the cornerstone of mechanical PPH treatment. Examples include the Bakri balloon and other commercial or improvised systems. The balloon is inserted into the uterus and inflated with saline, applying internal pressure to mechanically compress the bleeding sites within the uterine wall. Their widespread preference stems from a high success rate (often above 80%) and their relative ease of use, making them deployable by mid-level providers.
Non-pneumatic Anti-Shock Garments (NASG): A simple, life-saving technology consisting of neoprene segments applied firmly around the lower body. The NASG reverses hypovolemic shock, stabilizes vital signs, and shunts blood back to core organs (heart, brain) while transportation to definitive care or emergency intervention is organized. These are particularly vital in decentralized and low-resource settings.
Quantitative Blood Loss (QBL) Measurement Devices: Although diagnostic, devices like calibrated drapes, funnels, and weight-based collection systems are increasingly integrated into the treatment market. Accurate QBL measurement ensures timely treatment initiation, driving demand for all subsequent treatment devices.
Specialized Surgical Instruments: This niche includes tools for minimally invasive or direct surgical management, such as the B-Lynch suture kit or devices designed for temporary uterine artery occlusion.
Drivers and Current Dynamics
Global Maternal Health Initiatives: International bodies, including the WHO and the United Nations Population Fund (UNFPA), are aggressively promoting evidence-based PPH care, directly translating into institutional purchases of UBT and NASG kits across regional health systems.
The PPH Bundle Approach: Adoption of standardized, multi-step response protocols—known as "PPH Bundles"—in HICs has made mechanical devices mandatory steps in the care pathway. This shift from sequential steps to simultaneous intervention drives predictable, protocol-driven demand.
Rising Incidence of Risk Factors: The global increase in C-section rates (a major PPH risk factor) and the growing age of mothers in HICs contribute to higher PPH incidence, further increasing the addressable market size for treatment devices.
Decentralization of Births: The push for births in lower-level facilities (district and primary care hospitals), especially in LMICs, necessitates the use of simple, non-surgical PPH solutions like UBTs, as surgical expertise is often unavailable.
Market Size & Forecast
- The global postpartum hemorrhage treatment devices market was valued at USD 997.65 million in 2024 and is expected to reach USD 1,452.63 million by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 4.9%,
For More Information Visit https://www.databridgemarketresearch.com/reports/global-postpartum-hemorrhage-treatment-devices-market
Key Trends & Innovations
Innovation in PPH devices focuses heavily on optimizing delivery speed, reducing complexity, and improving safety.
1. Integration of QBL into Treatment Systems
The most significant trend is the mandatory integration of Quantitative Blood Loss (QBL) measurement. Hospitals are moving away from inaccurate visual estimation. New innovations combine collection drapes and weighing systems with the PPH treatment protocol itself, ensuring that therapeutic interventions (like UBT deployment) are triggered by objective, measured blood loss thresholds, saving critical minutes.
2. Self-Regulating and Automated UBTs
Traditional UBT systems require manual inflation, leading to inconsistent intrauterine pressure and potential user error. The future lies in self-inflating, pre-calibrated, or pressure-regulated balloons. These devices contain internal mechanisms or simple external gauges to ensure consistent, optimal pressure is applied to the uterine wall, maximizing efficacy and reducing the steepness of the user learning curve.
3. Single-Use, Pre-Sterilized, and Portable Kits
For rapid deployment, especially in emergency scenarios and low-resource settings, the market favors pre-packaged PPH Response Kits. These kits contain everything needed—from calibrated drapes and catheters to the UBT itself—in a single, sterile pack. This standardization is crucial for reducing delays during the "Golden Hour" of PPH management.
4. Advanced Hemostatic Materials
While less common due to strict regulatory hurdles, research is progressing on combining mechanical devices with hemostatic agents. This involves developing specialized coatings or materials for the balloon surface that promote clotting upon contact with the uterine wall, offering a synergistic mechanical and chemical solution.
Competitive Landscape
Competition in this market is bifurcated, existing between established MedTech firms competing on feature sets and quality in HICs, and high-volume, cost-effective manufacturers competing for global health procurement contracts.
Major Players and Strategies
Global MedTech Leaders (Cook Medical, Teleflex, BD): These firms leverage established global distribution channels and their extensive obstetric product lines. Their strategy is to offer premium, clinically validated UBTs and specialized surgical instruments, commanding high ASPs in developed markets.
Specialty Device Manufacturers (Local & Niche): Companies focusing exclusively on PPH, often developing simplified, ruggedized NASG or low-cost UBTs. Their success hinges on securing strong partnerships with global health NGOs (like UNFPA or USAID) to access volume tenders and humanitarian distribution networks.
Regional Generic Manufacturers: Significant competitive pressure comes from local manufacturers in India and China who produce highly cost-effective, but often less clinically documented, UBTs and other basic PPH instruments.
Key Competitive Strategies
Clinical Credibility and Guideline Inclusion: The ultimate competitive differentiator is robust clinical evidence (Level 1 RCTs). Inclusion in major professional society guidelines (ACOG, FIGO) or WHO/UNFPA best practices drives institutional procurement and market share.
Tiered Pricing and Global Access: Successfully navigating the market requires a dual pricing strategy: premium pricing in the U.S./Europe and highly discounted pricing for global public health tenders. This ensures brand presence and volume sales in high-burden regions.
Training and Education Services: Winning contracts often requires providing comprehensive simulation-based training and educational support. Companies that can demonstrate a commitment to improving user competency alongside device sales gain significant trust and long-term facility adherence.
Regional Insights
North America and Europe
These markets prioritize quality, standardization, and technology integration. Growth is driven by mandatory QBL protocols and the adoption of advanced, integrated kits. The focus is on the prevention and early detection side of PPH management, ensuring every facility adheres to evidence-based PPH bundles. High reimbursement rates support the use of premium devices.
Asia-Pacific (APAC)
APAC is the primary volume growth engine. Countries like India, China, and Indonesia have high birth rates and a significant PPH burden. The market demands robust, reusable, or very low-cost single-use devices. Strategic success requires local manufacturing, partnerships with regional governments for large-scale procurement, and adapting packaging for diverse local supply chains.
Africa and Latin America (LAMEA)
These regions face the greatest challenges in maternal health. The market is overwhelmingly driven by NGOs, philanthropic funding, and global aid organizations. The core demand is for Non-pneumatic Anti-Shock Garments (NASG) and affordable, simple UBT systems that can be used rapidly by first responders or in facilities with limited resources and frequent power outages.
Challenges & Risks
1. Inconsistent Protocol Adherence
The greatest risk is that the availability of devices does not equate to their effective utilization. Lack of standardized team training, insufficient PPH drills, and reluctance to abandon subjective blood loss estimation lead to delayed treatment and poor patient outcomes, undermining device investment.
2. The Pricing and Sustainability Conflict
The ethical imperative to supply life-saving devices at extremely low cost in LMICs clashes with the commercial need to fund sophisticated R&D and maintain high manufacturing quality (c-GMP). This fundamental dualism creates margin pressure and complicates the long-term sustainability of innovative device supply.
3. Regulatory Complexity and Off-Label Use
New innovations face a long and costly regulatory pathway, particularly in achieving FDA and CE mark approval. Simultaneously, in low-resource settings, providers often rely on off-label use of common items (e.g., condom catheters) as makeshift PPH tools, which presents a constant, low-cost competition to commercial devices.
4. Supply Chain Fragility
The global reliance on limited specialized manufacturing capacity for components, coupled with the need to distribute to remote, often challenging logistical locations (LMICs), exposes the supply chain to risks of disruption and stock-outs, which can have life-threatening consequences.
Opportunities & Strategic Recommendations
Stakeholder Group | Strategic Recommendation | Rationale |
|---|---|---|
Manufacturers | Develop and Market the PPH System, Not Just the Device. Focus R&D on integrated PPH kits combining UBT, QBL, and training modules into a single, comprehensive solution. | Drives institutional adoption through standardized protocols, offers a higher ASP, and creates a valuable, high-switching-cost system solution. |
Startups/Innovators | Pioneer Predictive and Non-invasive Diagnostics. Focus on simple, low-cost wearable devices that monitor vital signs (e.g., heart rate variability, skin temperature) to predict early signs of shock before blood loss is visually recognized. | Shifts the market from reactive treatment to proactive, personalized risk management, which is the next major frontier in maternal safety. |
Global Health Investors | Target NASG and QBL Manufacturing Scale-Up. Invest in organizations that can scale local or regional manufacturing of NASG devices and affordable QBL systems for bulk tender agreements. | These are the most critical, cost-effective technologies for immediate global impact in high-burden regions, guaranteeing massive volume sales through UN/NGO procurement. |
Hospital Systems (HICs) | Adopt Mandated PPH Simulation and Team Training. Integrate PPH device usage into mandatory, high-fidelity simulation drills for all OB/GYN, Anesthesia, and Nursing staff. | Improves provider competency, reduces procedural time delays, and ensures immediate, correct application of the devices during a crisis, validating the investment. |
Policymakers/Regulators | Create Accelerated Approval Pathways for Essential Maternal Devices. Establish a fast-track regulatory process for PPH devices deemed essential by the WHO for LMICs, separate from the full standard approval for HICs. | Encourages faster deployment of verified, life-saving technologies to the areas with the greatest unmet need, balancing speed and safety. |
Browse More Reports:
Global Intelligent Power Distribution Unit (PDU) Market
North America Surgical Power Tools Market
Global Non-Invasive Cancer Diagnostics Market
Global Cerebral Vasospasm Market
Middle East and Africa Diet and Nutrition Apps Market
Asia-Pacific Polyimide Films Market
Global Offshore Drilling Market
Global Paragonimiasis Treatment Market
Middle East and Africa Surgical Power tools Market
Europe Sustainable Aviation Fuel Market
Global Cardiovascular Ultrasound System Market
Global Uterine Polyps Drug Market
Global Dental Consumables Market
Global Amusement Parks Market
Global Polyurethane Additives Market
Global Bottled Cocktail Market
Global Self-Adhesive Tear Tape Market
Global Nursing and Residential Care Market
Global Titanium Dioxide Market
Europe Biopreservation Market
Asia-Pacific Personal Care Ingredients Market
Global Yeast Infection Market
Asia-Pacific Unmanned Ground Vehicle Market
Global Shale Gas Processing Equipment Market
Global Veterinary Blood Lactate Test Meter Equipment Market
Asia-Pacific Commercial Cleaning Equipment Market
Asia-Pacific Retail Analytics Market
Europe Weight Loss and Obesity Management Market
North America Biopreservation Market
Global Automotive Emission Test Equipment Market
North America Hydrographic Survey Equipment Market
Global Modular Liquid Crystal Polymer (LCP) Connectors Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com