Executive Summary
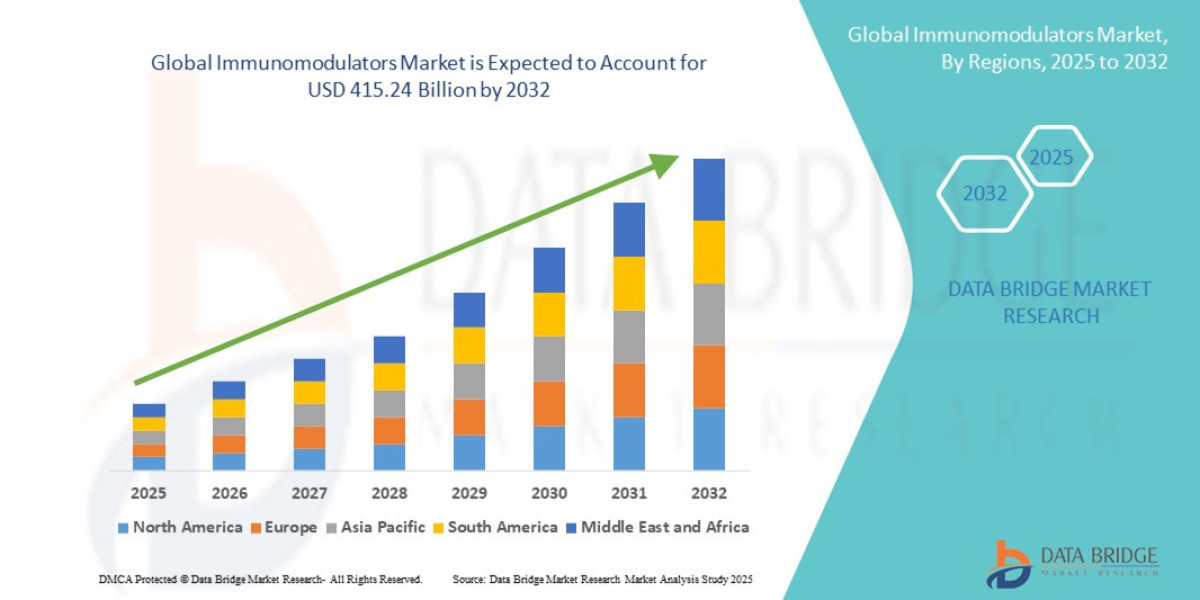
- The global immunomodulators market size was valued at USD 247.16 billion in 2024 and is expected to reach USD 415.24 billion by 2032, at a CAGR of 6.70% during the forecast period
Market Overview
The Immunomodulators Market covers all substances—biological or chemical—that affect the functioning of the immune system. These agents are categorized based on their mechanism of action: stimulating the immune response (Immunostimulants) or suppressing it (Immunosuppressants).
Key Segments by Drug Class
The market segmentation reflects therapeutic modality and mechanism:
Immunosuppressants (Largest Segment): Used to dampen excessive or undesirable immune responses.
Calcineurin Inhibitors (e.g., Cyclosporine, Tacrolimus): Primarily used in organ transplantation.
Corticosteroids: Broad-acting anti-inflammatories.
Biologic Drugs: Dominant and fastest-growing sub-segment, including TNF-alpha inhibitors (e.g., Humira, Remicade) and interleukin inhibitors, which target specific cytokines in autoimmune diseases.
Immunostimulants (Fastest Growing Segment): Used to enhance the immune system's ability to fight disease.
Immune Checkpoint Inhibitors (ICIs): Monoclonal antibodies (e.g., Keytruda, Opdivo) that block inhibitory receptors (PD-1/PD-L1, CTLA-4), releasing the immune system's brakes to attack cancer.
Vaccines: While technically immunomodulators, the market focus here is on novel therapeutic agents (e.g., cancer vaccines, adjuvant therapies).
Drivers and Current Dynamics
Core Market Drivers:
Rising Prevalence of Autoimmune Diseases: Global lifestyle changes and diagnostic improvements contribute to the growing incidence of chronic conditions like Psoriasis, Inflammatory Bowel Disease (IBD), and Multiple Sclerosis.
Success of Immuno-Oncology (IO): Checkpoint inhibitors have fundamentally transformed cancer treatment, driving massive revenue and R&D investment into combination therapies and new targets.
Maturation of Biologic Therapies: The therapeutic landscape continues to move away from small-molecule generics toward targeted biologics, offering superior efficacy and fewer systemic side effects, albeit at a higher cost.
Increasing Organ Transplant Procedures: Growing transplant success rates increase the long-term demand for effective, sustained immunosuppression regimens.
Current Dynamics: The market is characterized by a strategic tug-of-war between the launch of new, high-priced, targeted biologic and cell therapies and the rising pressure from biosimilars to established blockbuster anti-TNF therapies.
Market Size & Forecast
- The global immunomodulators market size was valued at USD 247.16 billion in 2024 and is expected to reach USD 415.24 billion by 2032, at a CAGR of 6.70% during the forecast period
For More information Visit https://www.databridgemarketresearch.com/reports/global-immunomodulators-market
Key Trends & Innovations
Innovation is focused on improving therapeutic specificity, reducing systemic side effects, and transforming non-responders into responders.
1. Next-Generation Checkpoint Inhibitors and Bispecifics
While PD-1/PD-L1 and CTLA-4 therapies are the standard, research is focused on new inhibitory targets (e.g., LAG-3, TIGIT) and agonistic targets (e.g., OX40, GITR) to stimulate T-cells. The most significant innovation is Bispecific Antibodies, which simultaneously target two different receptors, potentially improving efficacy and widening the therapeutic window, particularly in solid tumors.
2. Oral Small Molecules for Autoimmunity (JAK Inhibitors)
Janus Kinase (JAK) inhibitors represent a significant trend. These are orally administered small molecules that modulate intracellular signaling pathways, providing an alternative to injectable biologics. While offering patient convenience, their class-wide safety concerns (e.g., cardiovascular risk) necessitate careful patient selection, driving innovation toward more selective JAK inhibitors (e.g., targeting only JAK1).
3. Personalized Immunomodulation and Biomarker Identification
The efficacy of immunomodulators can vary dramatically among patients. A major trend is the integration of biomarkers (e.g., PD-L1 expression, TMB, MSI) to better select patients who will respond to IO therapies, improving cost-effectiveness. In autoimmunity, genetic and proteomic profiling helps differentiate disease subtypes and tailor immunosuppressant regimens.
4. Microbiome Modulation
The gut microbiome is recognized as a powerful modulator of the systemic immune response, influencing the efficacy of checkpoint inhibitors and the severity of autoimmune diseases. Research is accelerating into therapeutic strategies that use fecal microbiota transplantation (FMT) or specific microbial consortia to enhance the effectiveness and reduce the toxicity of established immunomodulators.
Competitive Landscape
The market is fiercely competitive, characterized by high barriers to entry due to R&D costs, complex clinical trials, and intellectual property protection, leading to domination by multinational pharmaceutical giants.
Major Players and Strategic Strategies
Pharmaceutical Blockbuster Holders (e.g., AbbVie, Johnson & Johnson, Pfizer): These firms derive significant revenue from legacy biologics. Their strategy focuses on Life Cycle Management (developing new delivery systems, indications) to fend off biosimilar erosion and acquiring next-generation IO assets to secure future growth.
Immuno-Oncology Leaders (e.g., Merck & Co., Bristol Myers Squibb, Roche): Dominating the fastest-growing IO segment. Their strategy is centered on combination therapy trials, expanding existing ICI approvals into dozens of tumor types, and aggressively pursuing novel co-stimulatory and co-inhibitory targets.
Biotech Specialists (e.g., Regeneron, smaller IO/Autoimmune startups): These companies focus on discovering highly specific novel targets (e.g., orphan receptors) or optimizing delivery methods. Their strategy is platform validation followed by high-value acquisition or exclusive partnership with a large pharma company.
The core competitive axis is shifting from patent protection of blockbuster drugs to the speed of biosimilar development and the demonstrated superior safety/efficacy of next-generation targeted biologics.
Regional Insights
Market size and drug utilization are closely linked to healthcare spending, regulatory speed, and therapeutic guidelines.
North America (Primary Revenue Driver and Innovation Hub)
Performance: The largest market, accounting for over 40% of global revenue. Characterized by high drug prices, rapid regulatory approval for breakthrough therapies (FDA), and extensive patient access to specialist care.
Opportunity: Continued growth in advanced biologic use and high R&D spend to maintain pipeline dominance.
Europe (Biosimilar Penetration and HTA Pressure)
Performance: A major market with high consumption, but growth is restrained by stringent Health Technology Assessment (HTA) agencies and single-payer systems that prioritize cost-effectiveness. This environment has led to the highest rate of biosimilar adoption globally.
Opportunity: Manufacturers need to focus on generating strong real-world evidence and pharmacoeconomic data to justify pricing and gain reimbursement across the fragmented European market.
Asia-Pacific (APAC) (Rising Demand and R&D Localization)
Performance: The fastest-growing region, fueled by rising disposable income, expanding healthcare access, and the increasing diagnosis of autoimmune diseases. Governments (especially China, Japan) are investing heavily to localize drug development and manufacturing.
Opportunity: Demand for both established generics/biosimilars (due to cost sensitivity) and cutting-edge IO therapies is accelerating. Strategic partnerships are required to navigate diverse regulatory pathways (e.g., China's NMPA).
Challenges & Risks
The complexity and cost of immunomodulators introduce significant market barriers and risks.
1. High Cost and Affordability Crisis
The high price tags of new biologics and checkpoint inhibitors place a severe strain on healthcare budgets. This necessitates complex negotiation with payers, limits patient access in lower-income countries, and drives the rapid development of biosimilar competitors.
2. Safety Profile and Immunogenicity
Immunomodulators carry inherent risks of serious side effects, including severe infections (due to broad immunosuppression) and life-threatening immune-related adverse events (irAEs) associated with ICIs. The development of anti-drug antibodies (immunogenicity) can also reduce drug efficacy over time, requiring close patient monitoring and therapeutic switching.
3. Biosimilar Erosion
The loss of patent protection for major blockbusters (e.g., Humira, Enbrel) leads to a rapid influx of lower-cost biosimilars, which pressures pricing across the entire therapeutic class and forces originators to rapidly innovate or shift their focus.
4. Regulatory and Trial Complexity in Combination Therapies
The increasing focus on IO combination therapies makes clinical trial design exponentially complex, often requiring larger patient populations and longer follow-up periods to establish safety and efficacy profiles, significantly increasing development cost and risk.
Opportunities & Strategic Recommendations
Strategic success in the immunomodulators market relies on innovation in targeting, combination, and value demonstration.
Strategic Recommendations for Stakeholders
Invest in Combination Therapy Optimization: For oncology, the greatest value is in identifying the optimal partners (e.g., novel agonists, anti-angiogenic agents) for PD-1/PD-L1 therapies, moving beyond current standard-of-care combinations to improve response rates in non-responders.
Focus on Autoimmunity Disease Modification: Shift R&D focus toward therapies that offer potential disease modification rather than just symptom management. This includes targeted cell therapies (e.g., regulatory T-cells) and antigen-specific tolerance induction strategies that "re-educate" the immune system.
Harness Predictive Biomarkers: Invest heavily in diagnostics and AI-driven analysis to develop robust companion diagnostics. This is crucial for both reducing the high cost of IO treatments (by screening out non-responders) and for justifying the price to payers based on demonstrated patient selection.
Adopt a Biosimilar Defense Strategy: Originator companies must accelerate the development of next-generation, differentiated products before patent expiration to mitigate biosimilar impact. Biosimilar developers should focus on early market entry and achieving interchangeability status with regulators to maximize market penetration.
Browse More Reports:
Global Clostridial Diseases Market
France Aluminum Pigments Market
Global Optical Spectrum Analyzer Market
Asia-Pacific Rice Husk Ash Market
Global Rugged Smartphones Sensors Market
Global Protein Labelling Market
North America Laminated Busbar Market
Global Behavioral Health Care Software and Services Market
North America Topical Corticosteroids Market
Global Dairy Desserts Market
Europe Genetic Testing Market
Global Tissue Banking Market
Global Xylose Market
Global Pulmonary Embolism Market
Middle East and Africa Healthcare Logistics Market
Middle East and Africa Rowing Boats and Kayaks Market
Europe Unmanned Ground Vehicle Market
Global Unidirectional Tapes Market
Global Voice Termination Market
Global Plant-Based Milk Market
Global Ion Milling System Market
Europe Hydrographic Survey Equipment Market
Global Wind Turbine Pitch System Market
Global Industrial Vehicles Market
Europe Data Integration Market
Asia-Pacific Fuse Market
Global Non Hodgkin Lymphoma Market
Global Dairy Flavours Market
Global Immune Health Supplements Market
Global Bone Conduction Hearing Aids Market
North America Plastic Compounding Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com